Apply appropriate primary dressing for permeability and non adherence to cover Talymed® such as Mepitel® One or Wound Veil. May fixate with Suture Strips in a "window pane" fashion as desired.
Overview of Application Technique

Manage infection and debride necrotic tissue prior to initiation of Talymed®. Clean wound with normal saline or sterile water.
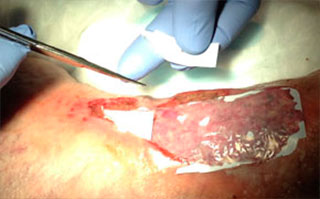
Apply Talymed® to wound bed. Conform or trim Talymed® as needed to approximate wound size and allow for slight wound margin overlap. Peri-wound overlap is desired and will not harm intact skin.
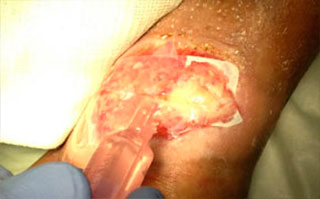
Apply saline, unless wound is heavily draining, conforming Talymed® to the wound bed using gauze or a sterile swab to assure intimate contact, full matrix integration and coverage of all aspects of wound bed and margin.
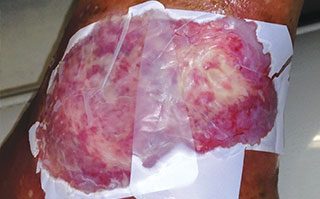
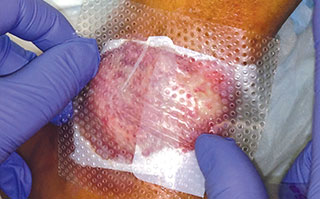
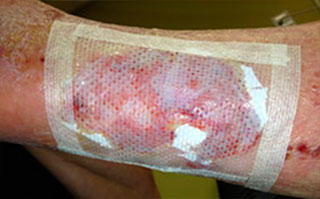
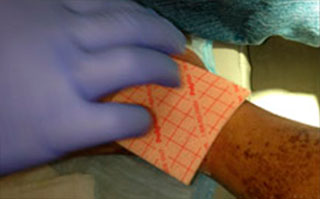
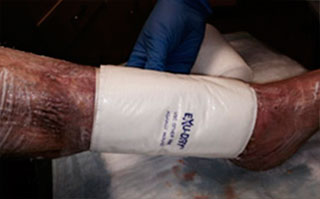
Apply appropriate secondary or retention dressing assuring moisture balance while maintaining a moist wound environment.
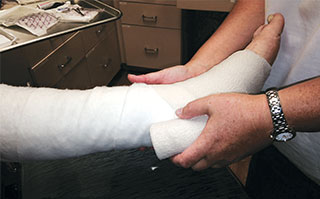
Apply adjunctive standard management protocols per etiology.
Please see complete application instructions below.
Easy-to-Use Wound Care
An advanced wound healing matrix, Talymed® provides easy-to-use wound care that is safe and effective for the management of wounds, including serious, chronic wounds. Talymed® offers these advantages:
- No pre-mixing or reagents
- No special storage conditions
- Few dressing changes
- Available in 10 cm x 10 cm, 5 cm x 5 cm, or 3 cm x 3 cm
Talymed® is packaged in a convenient blister pack and can be stored at room temperature for up to 3 years.
Talymed® Application Procedure
- Treat and manage acute infection and debride any necrotic /devitalized tissue, if present, prior to initiation of Talymed®.
- Gently cleanse wound bed with normal saline or sterile water.
- Open Talymed® blister pack using sterile or aseptic technique based on care setting and facility protocol.
- Use forceps or gloved hand to gently remove Talymed® from packaging and apply to wound bed.
- Apply Talymed® in a dry state, positioning for maximum wound coverage. Trim, position, and conform additional Talymed® as needed to assure coverage of the entire wound surface and wound margin allowing for a slight peri-wound margin overlap to facilitate contact with the basement membrane zone.Talymed® will not harm intact skin. If additional Talymed® is required for full coverage of the wound and margin, slightly overlap edges of the matrix to assure no gaps are present.
- Use drops of sterile water, saline, saline saturated gauze, or a moistened sterile swab to "pat" matrix in place and facilitate intimate contact, full matrix integration, and coverage of all aspects of wound bed and margin. Matrix will change from white to clear, signaling integration. Additional saline or sterile water may not be necessary, if wound is heavily draining or wound bed is very moist from cleansing and Talymed® conforms and integrates upon contact with the wound bed.
- Cover Talymed® with a non-adherent, permeable primary dressing such as Mepitel® One or ADAPTIC® and may fixate with suture strips using a "window-pane" technique if desired.
- Apply a secondary dressing of choice based on characteristics and level of drainage, assuring moisture balance and fluid handling capability. The secondary dressing may be changed or reinforced as needed. However, it is recommended to avoid disturbing Talymed® and the primary dressing for 5-7 days, or as clinical judgment determines.
- Talymed® should be left in place and applied at a frequency based on clinical judgment or once weekly for optimal results. It is recommended that debridement, other than hyperkeratotic wound margins that could potentially compromise epithelial migration, should be avoided during the course of therapy to allow for scaffolding to build.
- Employ adjunctive therapies such as compression, off-loading, and negative pressure wound therapy based on etiology, clinical judgment, and facility protocols.
- When performing dressing changes, employ gentle wound cleansing techniques in cleansing the wound bed prior to reapplying Talymed®.
- Reapply Talymed® by repeating previous application steps as required and continue until goal of therapy is achieved.
- During the first 1-2 weeks of therapy with Talymed®, an increase in drainage may or may not be observed. During week 3-4 of therapy a caramelized appearance of the wound bed may or may not be noted. If a caramelized appearance is noted, do not attempt to remove if the wound exhibits positive signs of healing (i.e. diminishing wound size, epithelialization).
Ensure Optimal Wound Healing

In a randomized, controlled study of 82 patients with venous leg ulcers unhealed after 3 months, standard care plus Talymed® showed superior healing rates as compared to standard care alone (P = .005).1
To learn more about Talymed's advanced wound healing properties, please contact us today.
Indications for Use:
Talymed® is indicated for the management of wounds including: diabetic ulcers, venous ulcers, pressure wounds, ulcers caused by mixed vascular etiologies, full thickness and partial thickness wounds, second degree burns, surgical wounds-donor sites/grafts, post-mohs surgery, post-laser surgery, and other bleeding surface wounds, abrasions, lacerations, traumatic wounds healing by secondary intention, chronic vascular ulcers, dehisced surgical wounds.
Safety Information:
PRECAUTIONS: There are no known contraindications to the use of Talymed®.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician or other licensed practitioner.
Manufactured by Marine Polymer Technologies, Inc. For instructions for use or additional product information, please contact us.
- 9,139,664
- 9,139,663
- 8,871,247
Talymed® - Ordering Information:
| Item # | Description | Matrix Size |
|---|---|---|
| 400-20 | Talymed 5 cm x 5 cm | 25 sq cm |
| 400-35 | Talymed 3 cm x 3 cm | 9 sq cm |
