Talymed® Enables Fast, Easy Wound Management
In clinical studies Talymed proved highly effective for managing serious, chronic wounds. Talymed is non-immunogenic with no known contraindications. To learn more, contact us today.
Long Standing Venous Leg Ulcer
A persistent venous leg ulcer treated with Talymed was healed by the third application.
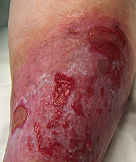
Prior to treatment
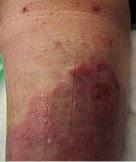
After three applications with Talymed
Diabetic Foot Ulcer
This patient's diabetic foot ulcer healed in two weeks following a single treatment with Talymed.
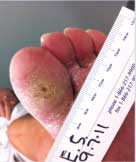
Prior to treatment
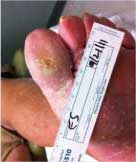
Healed after one treatment with Talymed
Severe Diabetic Foot Ulcer
Following Talymed applied three times in one month, this severe diabetic foot ulcer was healed.
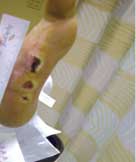
Prior to treatment
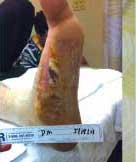
After one treatment with Talymed
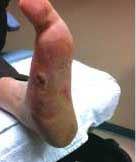
After two treatments with Talymed
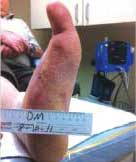
Healed after three treatments with Talymed

To learn more about Talymed's advanced wound healing properties, please contact us today.
Indications for Use:
Talymed® is indicated for the management of wounds including: diabetic ulcers, venous ulcers, pressure wounds, ulcers caused by mixed vascular etiologies, full thickness and partial thickness wounds, second degree burns, surgical wounds-donor sites/grafts, post-mohs surgery, post-laser surgery, and other bleeding surface wounds, abrasions, lacerations, traumatic wounds healing by secondary intention, chronic vascular ulcers, dehisced surgical wounds.
Safety Information:
PRECAUTIONS: There are no known contraindications to the use of Talymed®.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician or other licensed practitioner.
Manufactured by Marine Polymer Technologies, Inc. For instructions for use or additional product information, please contact us.
- 9,139,664
- 9,139,663
- 8,871,247
Talymed® - Ordering Information:
| Item # | Description | Matrix Size |
|---|---|---|
| 400-20 | Talymed 5 cm x 5 cm | 25 sq cm |
| 400-35 | Talymed 3 cm x 3 cm | 9 sq cm |
